Introduction To Medicinal Chemistry Solutions Pdf Download
Introduction
In the mid-seventies, major efforts were focused on the identification of new natural cannabinoids isolated from preparations of Cannabis sativa and of other subspecies and varieties, such every bit Cannabis indica and Cannabis ruderalis. The two most abundant and well-nigh therapeutically relevant components of the plants are (–)-trans-Δ9–tetrahydrocannabinol (Δ9–THC) and (-)-cannabidiol (CBD) (Effigy 1). Over these last two decades, the endocannabinoid organization (ECS) related to the furnishings of Cannabis sativa has existence emerging equally target of pharmacotherapy showing very considerable physiological significance (Mechoulam et al., 2014). This organisation includes two cannabinoid receptors (CB1 and CBii) and endogenous ligands named endocannabinoids (Matsuda et al., 1990; Munro et al., 1993). CBane receptor is abundant in the encephalon, but to a less extend in peripheral tissues. CB2 receptor is mainly expressed in allowed cells.
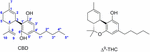
Figure 1. Cannabidiol (CBD) and (–)-trans-Δ9–tetrahydrocannabinol (Δ9–THC).
Δ9–THC is responsible for the psychoactive effects of Cannabis sativa mediated past the activation of CB1 receptor in the brain, whereas CBD is considered non-psychotropic. Currently, CBD is clinically used in association with Δix–THC in a cannabis-based preparation (Sativex®) that contains equimolar content of both for managing neuropathic symptoms associated with multiple sclerosis (Fernandez, 2016). CBD every bit a single drug is currently generating considerable interest due to its beneficial neuroprotective (Fernandez-Ruiz et al., 2013; Scuderi et al., 2014; Ibeas Bih et al., 2015), antiepileptic (Devinsky et al., 2015; Wright et al., 2015), hypoxia-ischemia (Lafuente et al., 2011; Mori et al., 2017), anxiolytic (Massi et al., 2013; Schier et al., 2014), antipsychotic (Bhattacharyya et al., 2010), analgesic (Maione et al., 2011), anti-inflammatory (Ruiz-Valdepeñas et al., 2011; Burstein, 2015), anti-asthmatic (Ribeiro et al., 2015; Vuolo et al., 2015), and antitumor properties (McAllister et al., 2011; Massi et al., 2013) among others(Mechoulam et al., 2007; Zhornitsky and Potvin, 2012; Renard et al., 2017; Watt and Karl, 2017). In 2016, GW pharmaceuticals reported the outset positive results of CBD (Epidiolex®) in phase Three clinical trials for treatment-resistant seizure disorders, including Lennox–Gastaut and Dravet syndromes. An overview of regulatory approvals and clinical trials of CBD has been recently published (Fasinu et al., 2016).
The molecular targets involved in the diverse therapeutic properties produced by CBD are however not very well-understood (Morales et al., 2017). Different Δnine–THC, CBD does not demark to the orthosteric binding site of the CB1 and CB2 cannabinoid receptors (McPartland et al., 2007). Despite this lack of orthosteric affinity, CBD has been shown to antagonize the effects of the CBone/CBii agonists CP–55,940 and WIN55212 at the mouse CBi and at the human CBii receptors (Pertwee et al., 2002; Thomas et al., 2007). Therefore, allosteric activity of CBD at these receptors has been hypothesized. In a recent written report, CBD was shown to be a negative allosteric modulator of Δ9–THC and the endogenous cannabinoid 2–AG providing a possible explanation for some in vivo CBD effects (Laprairie et al., 2015; Morales et al., 2016). CBD has besides been shown to modulate endocannabinoid tone past inhibiting the cellular uptake of the endocannabinoid anandamide (Leweke et al., 2012). This effect has been attributed to the fact that CBD competes with anandamide for binding to fatty acrid-binding proteins (FABPs) which are intracellular proteins involved in the transport of anandamide to its metabolic enzyme fatty acid amide hydrolase (FAAH) (Elmes et al., 2015). Other possible molecular targets of CBD have been explored. Modulation of the GPR55 receptor by CBD has been evaluated in unlike signaling pathway assays. CBD acts every bit an antagonist preventing [35South]GTPγS binding and Rho activation (Ryberg et al., 2007; Whyte et al., 2009; Ford et al., 2010), modulating Ca2+ mobilization (Lauckner et al., 2008) and β-arrestin recruitment (Yin et al., 2009). CBD has also been proposed equally an antagonist of the GPR18 cannabinoid receptor (McHugh et al., 2012, 2014). Sure actions of CBD such as anti-inflammatory and immunosuppressive effects announced to be partially mediated through the serotonin and adenosine receptors that are not considered part of the ECS. For instance, CBD acts as a total 5-HT1A agonist, 5-HT2A weak fractional agonist and a not-competitive 5HT3A antagonist (Russo et al., 2005; Yang et al., 2010; Rock et al., 2012). The ability of CBD to activate the A1A adenosine receptor has also been proposed (Gonca and Darıcı, 2014). Other molecular targets have also been studied, amongst them, the PPARγ nuclear receptors (O'Sullivan et al., 2009; Esposito et al., 2011; Scuderi et al., 2014), glycine receptors (Ahrens et al., 2009; Xiong et al., 2012), GABAA receptors (Bakas et al., 2016), and transient receptor potential (TRP) channels (De Petrocellis et al., 2011, 2012). Studies focused on the possible epigenetic regulation of skin differentiation genes past CBD revealed that CBD is a transcriptional repressor that tin can control prison cell proliferation and differentiation through Dna methylation (Pucci et al., 2013). Despite all of this data, the mechanistic bases for the furnishings of CBD remain complex.
Cannabidiol constitutes ane of the most important components of therapeutic interest from Cannabis sativa. However, unlike the numerous synthesized cannabimimetics generated to provide a synthetic culling to THC, CBD derivatives accept only been superficially explored. The purpose of this review is to provide a structural overlook at natural and synthetic CBD derivatives. Due to the fact that diverse molecular targets are involved in the therapeutic properties produced by CBD, nosotros associated CBD structures to their biological targets. Thus, this review is intended to be a useful tool especially for medicinal chemists.
The basic construction of the CBD derivatives described in this review consists of 5-alkyl resorcinols substituted in position ii by a propenylcyclohexene. Structural modifications on the alkyl side-chain, on the propenylcyclohexene, and substitution of the phenolic hydroxyl groups are concerned. Quinone CBD analogs are also included in this nomenclature as far as their structures are closely related to CBD.
Natural Cannabidiol Derivatives
In a recently published review dedicated to the diversity of cannabis phytocannabinoids, the authors updated the inventory of naturally occurring CBD derivatives (Hanuš et al., 2016). Herein, nosotros are associating these structures to their possible molecular targets.
Of the over 100 natural cannabinoids identified in Cannabis Sativa, seven take been classified as CBD-type compounds including CBD (Figure 2) (ElSohly and Slade, 2005; ElSohly and Gul, 2014; Aizpurua-Olaizola et al., 2016). All of them have the same accented configuration than CBD; they are 5′-methyl-2′-(prop-one-en-2-yl)-1′,2′,3′,4′-tetrahydro-[i,ane′-biphenyl]-2,6-dioles retaining the trans-(oneR,half-dozenR) configuration. Cannabidiolic acrid (CBDA) and cannabidivarinic acid (CBDVA-C3) are C3′-carboxylic derivatives, whereas cannabidiorcol (CBD-C1), cannabidiol-C4 also named as nor-cannabidiol (CBD-C4), and cannabidivarin (CBDV) differ from CBD by the length of their C4′-side chain. Cannabidiol monomethyl ether (CBDM), the C6′-methoxy CBD analog, was as well isolated from the establish. Despite the potential therapeutic interest of these naturally occurring CBD derivatives, only a few related pharmacological studies accept been reported (Table 1). Like most non-steroidal anti-inflammatory drugs, CBDA is characterized by a carboxylic group resulting in a selective inhibition of cyclooxygenase-2 (Takeda et al., 2008). CBDA does not have effect on anandamide inactivation in FAAH assays (Inhibition of [14C]-anandamide uptake: IC50 > 50 μM) contrary to CBD (IC50 = 28 μM) (Bisogno et al., 2001; Ligresti, 2006). Other molecular targets proposed for CBDA include GPR55 (Anavi-Goffer et al., 2012) and TRPA1 with moderate activity (De Petrocellis et al., 2011). CBDA has been shown to be an inhibitor of jail cell migration in the highly aggressive man chest cancer MDA-MB-231 by alteration of Rho GTPase activity (Takeda et al., 2012). CBDV, the C4′-propyl analog of CBD, displays very weak affinity for CB1 and CBii receptors (Hill et al., 2013; Rosenthaler et al., 2014), whereas it has been reported to inhibit the action of the putative endogenous ligand LPI in hGPR55-HEK293 cells (Anavi-Goffer et al., 2012). CBDV also targets the human TRPA1 channel (De Petrocellis et al., 2011, 2012). In several animal seizures models, CBDV exerted notable anticonvulsant furnishings without affecting normal motor function (Hill et al., 2012). The mechanisms through which CBDV exerts its antiepileptic furnishings are uncertain (Jones and Whalley, 2015). CBDV is currently in Stage Two clinical trials equally an antiepileptic drug under the name GWP42006.1
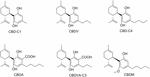
Figure two. Natural phytocannabinoid CBD analogs.
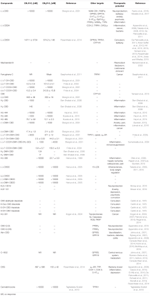
Table 1. CB1/CB2 cannabinoid receptor binding, molecular targets and therapeutic potential of CBD derivatives.
2 aromatic analogs of CBD accept been isolated from Lebanese hashish (ElSohly and Slade, 2005): cannabinodiol (CBND-C5), and cannabinodivarin (CBND-C3) (Figure iii) whose structural elucidation required their total synthesis (Robert et al., 1977). CBND-C5 found in the establish'southward flowers in low concentration, is considered a product of CBD photochemical conversion.
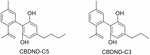
Effigy iii. Aromatic analogs of CBD.
The conversion of CBD into human being metabolites has been the subject of a contempo interesting review (Ujváry and Hanuš, 2016). CBD biotransformation shows considerable species variability. The main biotransformation, including hydroxylation and oxidation, involves the CYP450 enzyme family. While 7-hydroxy-CBD (vii-OH-CBD) derivatives are found in low concentration, the most abundant metabolites are hydroxylated vii-carboxylic acid derivatives of CBD (7-COOH-CBD, Figure 4). Glucuronidation of CBD seems to oftentimes occur at the phenolic oxygen (Figure 4). Another cannabinoid metabolite, the and then called cannabielsoin (CBE), has been identified in plants as a product of photo-oxidation from CBD and CBDA (Shani and Mechoulam, 1974; Ujváry and Hanuš, 2016), or by biotransformation using tissue cultures under normal growth conditions (Hartsel et al., 1983; Yamamoto et al., 1991). CBE was also identified equally a metabolite in guinea pigs, mice, rabbits, and rats (Yamamoto et al., 1991). Despite the fact that CBD metabolites have been the subject of many studies, few in vivo studies take been published. Therefore, their therapeutic benefits remain to be established.
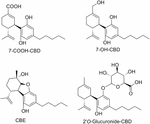
FIGURE four. Selected metabolites of CBD.
Beyond the Cannabis plant, other naturally occuring products have been reported to interact with the ECS (Gertsch et al., 2010). Nonetheless, only few of them are CBD-based compounds. Isolation and label of (+)-trans-hexahydrodibenzopyrans from the stalk bawl of the Amazonian liana Machaerium multiflorum Spruce led to the identification of the CBD related structures machaeridiols A, B, and C (Figure 5) (Muhammad et al., 2003). The total synthesis of these compounds via an efficient highly regio- and stereoselective approach has also been described (Huang et al., 2007). Although their activity at the CBi and CBtwo cannabinoid receptors has not been reported, these compounds displayed antimicrobial, antifungal, and antiparasitic activity in diverse in vitro assays (Muhammad et al., 2003). Machaeridiol B stands out equally the most potent inhibitor against Plasmodium falciparum [chloroquine-sensitive (D6) and chloroquine-resistant (W2) clones] and Leishmania donovani with IC50 values in the depression micromolar range (Table i).
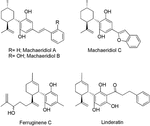
Effigy 5. Cannabidiol (CBD)-related Machaerium multiflorum, Rhododendron ferrugineum L. and, Lindera umbellata Thunb. compounds.
Ferruginene C, a methylpentanol derivative of CBD (Effigy 5), was recently isolated from the leaves of Rhododendron ferrugineum L. every bit a mixture of diastereoisomers (Seephonkai et al., 2011). Ferruginene C has been shown to be cytotoxic in the HL-sixty cancer jail cell-line (IC50 13.seven μM) with selectivity toward non-cancerous cell-line. It binds weakly to CBtwo and TPRV1 receptors, just it did not evidence meaning analogousness for CBi and v-HT1A receptors (Table 1).
Fifty-fifty though linderatin (Figure 5), isolated from fresh leaves of Lindera umbellata Thunb. (Tanaka et al., 1984), is not considered a phytocannabinoid (Hanuš et al., 2016), it is interesting to include in the present review since closely related to CBD. No biological information have been reported and so far.
Synthetic Cbd Analogs
Due to the promising therapeutic furnishings of CBD in a wide diverseness of diseases, constructed CBD derivatives take attracted the attention of drug discovery programs in both manufacture and academia with the aim to improve the authority, efficacy, or pharmacokinetic properties of this interesting phytocannabinoid.
Synthetic approaches for unlike CBD metabolites such as 7-COOH-CBD or 7-OH-CBD (Figure 4) accept been reported (Tchilibon and Mechoulam, 2000; Mechoulam and Hanuš, 2002). Moreover, structural modifications on different pharmacophoric positions such as the lipophilic side concatenation, the phenolic hydroxyl groups or the C7-methyl have been widely accomplished. In add-on to the (-)-CBD enantiomers, the (+)-CBD derivatives [(+)-CBD depicted in Effigy 6] have also been synthesized and pharmacologically evaluated (Bisogno et al., 2001; Fride et al., 2004; Hanus et al., 2005). Measurements of the binding affinities of these compounds for the CBane and CBtwo cannabinoid receptors yielded unexpected outcomes. Contrary to the naturally occurring (-)-CBD analogs, which showed no orthosteric affinity, near of the compounds in the (+)-CBD series bind to both receptors displaying selectivity toward CB1 (Table 1).
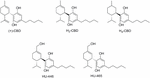
FIGURE vi. (+)-CBD and hydrogenated CBD derivatives.
Hydrogenation of CBD yielded the dihydro- and tetrahydro-cannabidiol derivatives Htwo-CBD and H4-CBD (Effigy 6) (Ben-Shabat et al., 2006). Their effects on the production of reactive oxygen intermediates, nitric oxide, and tumor necrosis factor showed their anti-inflammatory chapters. In contrast to CBD, H2-CBD, and H4-CBD have analogousness for the cannabinoid CBane receptor (Table 1). Additionally, the (-)- and (+)-dihydro-7-hydroxy-CBD enantiomers (HU-446 and HU-465, Figure six) accept recently been synthesized and biologically characterized in an inflammatory model of encephalitogenic T cells (Kozela et al., 2015). Both compounds showed anti-inflammatory potential in inflammatory and autoimmune diseases models. Yet, only the (+)-enantiomer (HU-465) displays analogousness for the cannabinoid CBane and CB2 receptors (Table i).
1′,1′ -Dimethylheptyl-CBD Derivatives
Taking into business relationship that exchange of the pentyl chain of Δ9–THC by a 1′, one′-dimethylheptyl (DMH) lipophilic alkyl chain resulted in more active compounds than natural Δ9–THC (Mechoulam et al., 1988), a similar arroyo was performed for the CBD scaffold (Mechoulam et al., 1990; Hanus et al., 2005) (Table ane). Thus, the synthesis of DMH-CBD derivatives, such as DMH-CBD, HU-320, DMH-CBDD, and seven-OH-DMH-CBD (Figure 7) accept been reported by Mechoulam and coworkers (Leite et al., 1982; Hanus et al., 2005). Introduction of the DMH alkyl chain in the (-)-DMH-CBD series did not modify the lack of CBone and CBii receptor analogousness except for (-)-7-OH-DMH-CBD that moderately binds to CB2 (Table i) (Bisogno et al., 2001). However, in the case of the (+)-DMH-CBD series, the presence of the DMH alkyl concatenation improved both CBone receptor affinity compared to (+)-CBD (Tabular array 1). (-)-DMH-CBD analogs have displayed anxiolytic, analgesic, anti-inflammatory, or antiproliferative effects in diverse assays (Burstein, 2015). For instance, (-)-DMH-CBD has shown anti-inflammatory and antiproliferative properties in man acute myeloid leukemia, microglial or encephalitogenic T cells (Juknat et al., 2016). The carboxylic acid HU-320 produced stiff anti-inflammatory and immunosuppressive effects in an in vivo model of collagen-induced arthritis (Sumariwalla et al., 2004). Interestingly, (-)-7-OH-DMH-CBD exhibited potent inhibition of electrically evoked contractions of the mouse vas deferens that was not mediated through CB1, CB2, TRPV1, opioid, or α2-adrenergic receptors (Fride et al., 2005; Pertwee et al., 2005).
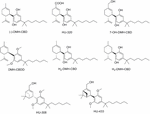
Figure 7. Dimethylheptyl (DMH)-CBD derivatives.
Every bit previously mentioned for the pentyl CBD derivatives, hydrogenation of DMH-CBD has been studied (Ben-Shabat et al., 2006). Partial hydrogenation gave H2-DMH-CBD (Figure 7) equally the major epimer (hydrogenation at C8) with small amounts of the hydrogenated C1 epimer existence obtained. Full hydrogenation allowed the formation of H4-DMH-CBD (Effigy 7). These hydrogenated compounds, which bind to the CB1 receptor with analogousness constants in the nanomolar range, displayed weak anti-inflammatory effects when compared to CBD or DMH-CBD.
The pinene dimethoxy-DMH-CBD derivative HU-308 (Figure 7) was identified decades ago every bit a potent peripheral CB2-selective agonist (Mechoulam et al., 1990; Hanus et al., 1999). HU-308 has shown very interesting properties such every bit anti-inflammatory, analgesic, neuroprotective or antitumor effects, and has been used every bit a pharmacological tool in numerous cannabinoid studies contributing to the progress in this field (east.g., Hanus et al., 1999; Ofek et al., 2006; Rajesh et al., 2007a,b; Burstein, 2015). More recently, the efficacy of HU-308 and HU-433, two enantiomers, has been tested in ovariectomy-induced bone loss and ear inflammation (Smoum et al., 2015) showing an inverse relationship between binding affinity and biological say-so.
Other Modifications on the C4′-Alkyl Concatenation
In society to ameliorate oral bioavailability and solubility issues, a novel series of CBD analogs have recently been synthesized (Kinney et al., 2016) (Effigy viii). Structural modifications at the pharmacophoric lipophilic chain allowed fine-tuning the "drug-likeness" of this scaffold by variation of different physicochemical parameters such as the number of hydrogen bond donors, acceptors, and polar surface area. Amidst these new derivatives depicted in Figure 8, KLS-13019 stands out as beingness 50-fold more potent and more than than 400-fold safer than CBD preventing damage to hippocampal neurons induced by ammonium acetate and ethanol with improved oral bioavailability compared to CBD (Kinney et al., 2016).
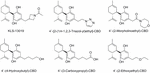
Figure viii. Cannabidiol analogs modified on the C4′-alkyl chain.
Halogenated CBD Derivatives
Structural modifications of CBD include halogenated substituents on the phenol ring. The first reported halogenations occurred at the 3′ and/or v′ positions by chlorine, bromine or iodine substitution, assuasive the preparation of 3′-Cl-CBD, 3′,5′-diCl-CBD, 3′-Br-CBD, 3′,5′-diBr-CBD, 3′-I-CBD, and 3′,5′-diI-CBD (Figure 9) (Usami et al., 1999). These halogenated compounds were evaluated in murine models of barbiturate-induced sleep prolongation, electroshock-induced seizures and locomotor activity resulting in activity similar to CBD for the monohalogenated analogs, whereas the dihalogenated derivatives displayed lower activity (Table 1).
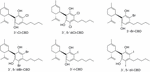
FIGURE 9. Chlorinated, brominated, and iodinated CBD derivatives.
The synthesis and pharmacological evaluation of three new fluorine halogenated CBD derivatives accept been reported (Breuer et al., 2016). Two of these were fluorinated at the cyclohexenyl ring substituent (Effigy 10: HUF-102 and HUF-103), and the third ane was fluorinated at the phenol ring (HUF-101). HUF-101 displayed the about promising results in four mice behavioral assays (elevated plus-maze, forced swimming test, prepulse inhibition, and marble burial test) that target anxiolytic, antidepressant, antipsychotic and anticompulsive activity respectively. HUF-101 may be an interesting image for further evolution since information technology showed higher potency than CBD in the animal assays cited higher up. In these tests, HUF-102 did not prove action at the doses tested (1–10 mg/kg), whereas HUF-103 showed moderate to depression activity compared to HUF-101.
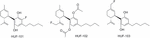
FIGURE 10. Fluorinated CBD derivatives.
Modifications on the Hydroxyl Groups
Modifications on the resorcinol hydroxyl groups have been explored. Computational studies suggested that the removal of one of the CBD hydroxyl groups may enable the ligand to accomplish the CB1 bounden site (Reggio et al., 1995). Thus, desoxy-CBD represented in Effigy 11 was synthesized and evaluated. Pharmacological data for desoxy-CBD corroborated the computational studies showing CB1 fractional agonism in the mouse vas deferens assay.
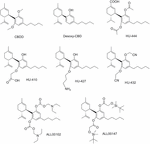
Figure 11. Cannabidiol derivatives modified at the hydroxyl groups.
Dissimilar inquiry groups have developed acetylations and alkylations at i or both phenolic hydroxyls. For instance, the dimethylated CBD derivative named CBDD (Figure 11), as well every bit the monomethylated derivative (CBD-2′-monomethylether o O-methylcannabidiol) revealed higher dominance and selectivity as 15-lipoxygenase inhibitors compared to CBD (Takeda et al., 2009, 2011). Consequently, the resorcinol moiety seems to be a determinant for the action in this target. Further studies performed with CBDD advise that this compound is not only a potential prototype for atherosclerosis handling, just also a pharmacological tool to study the mechanisms of body weight regulation (Takeda et al., 2015). Other alkylations on the phenolic hydroxyl group have been reported such as O-propyl- and O-pentylcannabidiol that take been structurally characterized but no pharmacological information have been described and then far (Hendricks et al., 1978).
Cannabidiol derivatives bearing one or both hydroxyl substitutions have been reported in the patent literature to be active as anti-inflammatory agents (Mechoulam et al., 2008). Selected examples disclosed in this patent (HU-410, HU-427, and HU-432) are depicted in Effigy 11. It is interesting to highlight that some of these compounds present improved solubility, stability and bioavailability parameters when compared with CBD. Likewise, the non-CB1, non-CB2 ligand HU-444 has shown anti-inflammatory properties in vitro and in vivo in a murine model of collagen-induced arthritis (Haj et al., 2015).
In addition, the in vivo anticonvulsant activeness of four diacetylated-CBD analogs (CBD-aldehyde-diacetate, 6-oxo-CBD-diacetate, six-hydroxy-CBD-triacetate, and 9-hydroxy-CBD-triacetate, Figure 12) was demonstrated in a mouse model (Carlini et al., 1975). Their effects against maximal electroshock convulsions, potentiation of pentobarbital sleeping-time and reduction of spontaneous motor activity were evaluated obtaining significant anticonvulsant effects at high doses. It is noteworthy that the safety, efficiency, and dominance of these four compounds were lower than that of CBD in the same assays.
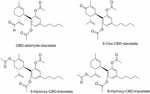
Figure 12. Diacetylated-CBD analogs.
At that point it is interesting to mention that these diacetate CBD derivatives could take been considered as prodrugs. Because that CBD is quickly distributed in adipose tissues and it undergoes a CYP3A- and CYP2C- dependent first-pass metabolism to give 7-hydroxy-CBD (Fasinu et al., 2016), a prodrug concept could exist very useful. Therefore, the phenyl acetate groups could exist deacetylated to give CBD. The pharmaceutical company, AllTranz, now called Zyberba Pharmaceutics, adult transdermal solutions of CBD-esters and -carbonates amongst others. The dicarbonate All00102 and the diglicolate AL00147 shown in Figure 11 are two examples disclosed in a AllTranz'southward patent (Stinchcomb et al., 2009). Another visitor, Kalytera Therapeutics is currently undertaking the preclinical stage of K-1012, a bi-phosphate derivative of CBD designed equally a prodrug indicated for acute respiratory distress syndrome.2
Quinone Derivatives of CBD
The quinone derivative of CBD, HU331, was first synthesized in Mechoulam et al. (1968) past oxidation of CBD. HU331 has been suggested to be a CBD metabolite having inhibitory effect on cytochrome P450 (Bornheim and Grillo, 1998). It was non until Kogan et al. (2004) that the antineoplastic activity of HU-331 was reported. HU-331 was very constructive in reducing growth of human colon carcinoma HT-29 cells in nude mice. The mechanism past which HU-331 acts equally an antitumor agent is contained of the CBone and CB2 cannabinoid receptors. HU-331 does not promote cell expiry via cell cycle arrest, cell apoptosis, or caspase activation. Extensive studies accept shown that HU-331 anticancer backdrop were due to selective inhibition of the ATPase function of homo topoisomerase IIα (Kogan et al., 2007; Peters and Kogan, 2007; Regal et al., 2014). Thus, HU-331 with a selective topoisomerase inhibition is expected to take less off-target toxicity than doxorubicin which antitumor activity is mediated through numerous mechanisms, such as apoptosis, absconding of the prison cell wheel, activation of caspases, generation of ROS, and inhibition of both topoisomerases among others.
Structural modifications realized on the substituents of HU-331 led to the benzoquinones having anti-proliferative activity against diverse cancer cell lines (Petronzi et al., 2013). Unlike HU-331, benzoquinone mechanism of activity involves caspase activation, poly-(ADP-ribose)-polymerase (PARP) protein cleavage, and reactive oxygen species (ROS) production. These data show the influence of CBD construction compared to the quinone cadre on the processes producing anticancer furnishings.
A contempo patent from VivaCell Technology discloses HU-331 analogs which act as PPARγ agonists showing a neuroprotective contour in different models (Appendino et al., 2015). The disclosed quinones are substituted in position 3′ by different amines or carboxylates that were synthesized by amination of CBD or esterification of CBDA respectively. Compounds CBD-Q (V) and CBD-Q (Eight) illustrated in Figure 13 are representative of the HU-331 analogs.
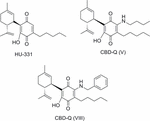
FIGURE xiii. Quinones related to CBD.
Miscellaneous CBD Derivatives
Aberrant cannabidiol (Abn-CBD) (Razdan et al., 1974), a not-psychoactive synthetic regioisomer of CBD (Figure 14), has been the subject area of numerous studies that accept shown Abn-CBD therapeutic potential as a vasodilator (Johns et al., 2007), antibacterial (Appendino et al., 2008), antidiabetic (McKillop et al., 2016), or anti-colitis agent (Krohn et al., 2016). Recently, ii molecular targets, GPR55 and GPR18, have been identified for Abn-CBD (Johns et al., 2007; Ryberg et al., 2007; Console-Bram et al., 2014). Abn-CBD stimulated [35Due south]GTPγS bounden at GPR55 (Oka et al., 2007) and increased calcium mobilization and ERK1/2 phosphorylation at GPR18 (Console-Bram et al., 2014).
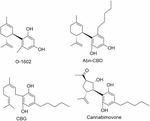
FIGURE 14. Miscellaneous CBD derivatives.
The synthetic cannabinoid O-1602 that does not demark significantly to CB1 or CB2 receptors, stimulates GTPγS activation in membranes from human recombinant GPR55-expressing cells (EC50 = 1.4 nM) (Johns et al., 2007; Console-Bram et al., 2014). In vivo, O-1602 showed anti-inflammatory activity in mice with cerulein-induced astute pancreatitis characterized past an increased expression of GPR55 receptor (Li et al., 2013). O-1602 has as well been shown to increment levels of GPR18-mediated MAPK activeness and calcium mobilization, merely not β-arrestin signaling, thus supporting that O-1602 acts equally a biased-agonist at GPR18 (Console-Bram et al., 2014). Data have been reported suggesting the therapeutic potential of O-1602 for diseases related to the key nervous arrangement (Ashton, 2012), or to metabolic diseases (Romero-Zerbo et al., 2011).
Another minor component of Cannabis sativa is cannabigerol (CBG) (Gaoni and Mechoulam, 1971). Structurally, CBG can be considered the cyclohexenyl-opened analog of CBD. Different therapeutic applications accept been proposed for CBG, more recently CBG has been shown to have antibacterial action (Appendino et al., 2008), antidepressant-like action (El-Alfy et al., 2010), and anti-inflammatory properties for bowel disease (Borrelli et al., 2013). Molecular targets of CBG include the αtwo adrenergic receptor, TRP channels, cyclooxygenase (COX-i and COX-2) enzymes, as well equally the 5-HT1A and cannabinoid receptors (Cascio et al., 2010; De Petrocellis et al., 2011; Ruhaak et al., 2011). Cannabimovone is one of the latest natural phytocannabinoids that has been extracted from a cultivar of hemp rich in CBD (Taglialatela-Scafati et al., 2010). The terpenoid structure of cannabimovone replaces the cyclohexenyl band of CBD by a functionalized cyclopentane including four face-to-face stereocenters. Its total synthesis has been reported very recently (Carreras et al., 2016). Cannabimovone is devoid of CBi and CB2 activity, whereas information technology is a weak TPRV1 agonist.
Conclusion
A meaning amount of preclinical data has shown the high therapeutic potential of CBD peculiarly in inflammatory mouse models. Co-ordinate to ClinicalTrials.gov records, CBD is currently tested in clinical phases for unlike inflammatory diseases. The results of the first clinical study of CDB for the treatment of inflammatory bowel have been published very recently. Unfortunately, the effects of CBD on Crohn'southward disease were ineffective in a randomized placebo-controlled trial on xx patients probably due to low used doses (Naftali et al., 2017). The potential antiepileptic effects of CBD in patients suffering seizures associated with Lennox–Gastaut syndrome and in children and young patients with Dravet syndrome are currently on-going. The research has tended to focus on CBD therapeutic applications. Less attention has been paid to the therapeutic utility of CBD derivatives.
Despite the identifications of CBD metabolites and naturally occurring CBD analogs, in general, their pharmacological properties have not been extensively studies. In what concerns synthetic CBD-based compounds, several of them have shown interesting pharmacological properties but none has been introduced into clinical trials yet. In a pharmacological signal of view, whereas CBD does non have affinity for both classical CB1 and CB2 cannabinoid receptors, about of (+)-CBD derivatives practice bind to CB1 and/or CB2 receptors. Others, such equally Abn-CBD, O-1602, CBG, cannabimovone, ferruginene C, (-)-CBDV, and (-)-CBDA, have shown activity at other receptors including TPRV1, GPR35 and/or GPR18 receptors, or enzymes such as COX-ii. A limitation of the development of CBD synthetic derivatives probably resides in the lack of a unique mutual molecular target.
In future therapeutic development of CBD derivatives, it will be prudent to accept into business relationship some structural considerations around the CBD scaffold. One of them is the possible atropisomerism effectually the phenyl–hexenyl bail. Ortho-substitution on the phenyl ring could have stereochemical consequences generating hindered rotation of the phenyl–hexenyl bond due to steric or electronic constraints, generating 2 isolable conformers in the case of ho-hum interconversion (Berber et al., 2014; Flos et al., 2016). Thus, information technology is necessary to consider the implication of a possible atropoisomerism for new CBD analogs discovery (Clayden et al., 2009).
The complexity of the pharmacological processes of CBD and CBD analogs suggest that a improve understanding of their mechanism of action is required to devise successful synthetic CBD-based drug therapies.
Author Contributions
PM, PHR, and NJ substantially contributed to the redaction of the manuscript. And so, they all approved the manuscript to be published.
Funding
Financial back up was provided by Castilian Grants from Ministerio de Economía y Competitividad SAF2015-68580-C2-2-R (MINECO/FEDER) (NJ) and NIH grants RO1 DA003934 and KO5 DA021358 (PR).
Conflict of Involvement Argument
The authors declare that the research was conducted in the absence of any commercial or fiscal relationships that could be construed as a potential disharmonize of interest.
Footnotes
- ^ClinicalTrials.gov. A Report of GWP42006 in People With Focal Seizures. https://clinicaltrials.gov/ct2/show/NCT02369471 (accessed September 22, 2016).
- ^https://kalytera.co/programs/preclinical/ (accessed June viii, 2017).
References
Ahrens, J., Demir, R., Leuwer, M., De La Roche, J., Krampfl, K., Foadi, N., et al. (2009). The nonpsychotropic cannabinoid cannabidiol modulates and direct activates alpha-i and alpha-1-beta glycine receptor part. Pharmacology 83, 217–222. doi: 10.1159/000201556
PubMed Abstruse | CrossRef Full Text | Google Scholar
Aizpurua-Olaizola, O., Soydaner, U., Öztürk, East., Schibano, D., Simsir, Y., Navarro, P., et al. (2016). Evolution of the cannabinoid and terpene content during the growth of cannabis sativa plants from different chemotypes. J. Nat. Prod. 79, 324–331. doi: 10.1021/acs.jnatprod.5b00949
PubMed Abstruse | CrossRef Full Text | Google Scholar
Anavi-Goffer, South., Baillie, 1000., Irving, A. J., Gertsch, J., Greig, I. R., Pertwee, R. Chiliad., et al. (2012). Modulation of L-α-lysophosphatidylinositol/GPR55 mitogen-activated poly peptide kinase (MAPK) signaling past cannabinoids. J. Biol. Chem. 287, 91–104. doi: 10.1074/jbc.M111.296020
PubMed Abstract | CrossRef Total Text | Google Scholar
Appendino, G., Cabello de Alba, K. L. B., and Munoz Blanco, E. (2015). Preparation of novel cannabidiol quinone derivatives as PPARγ agonists. International Patent No WO2015/158381. Washington, DC.
Appendino, G., Gibbons, S., Giana, A., Pagani, A., Grassi, G., Stavri, 1000., et al. (2008). Antibacterial Cannabinoids from Cannabis sativa : a structure-activeness study. J. Nat. Prod. 71, 1427–1430. doi: 10.1021/np8002673
PubMed Abstract | CrossRef Total Text | Google Scholar
Ashton, J. C. (2012). The atypical cannabinoid O-1602: targets, actions, and the central nervous organization. Cent. Nerv. Syst. Agents Med. Chem. 12, 233–239. doi: ten.2174/187152412802430156
PubMed Abstruse | CrossRef Full Text | Google Scholar
Bakas, T., Devenish, S., Van Nieuwenhuizen, P., Arnold, J., McGregor, I., and Collins, M. (2016). "The actions of cannabidiol and 2-arachidonyl glicerol on GABA-A receptors," in Proceedings of the 26th Annual Symposium on the Cannabinoids, International Cannabinoid Research Society, Bukovina, 28.
Ben-Shabat, Southward., Hanuš, L. O., Katzavian, Yard., and Gallily, R. (2006). New cannabidiol derivatives: synthesis, binding to cannabinoid receptor, and evaluation of their antiinflammatory activity. J. Med. Chem. 49, 1113–1117. doi: 10.1021/jm050709m
PubMed Abstract | CrossRef Total Text | Google Scholar
Berber, H., Lameiras, P., Denhez, C., Antheaume, C., and Clayden, J. (2014). Atropisomerism about aryl-csp3 bonds: the electronic and steric influence of ortho -substituents on conformational exchange in cannabidiol and linderatin derivatives. J. Org. Chem. 79, 6015–6027. doi: 10.1021/jo5006069
PubMed Abstract | CrossRef Full Text | Google Scholar
Bhattacharyya, S., Morrison, P. D., Fusar-Poli, P., Martin-Santos, R., Borgwardt, Southward., Winton-Chocolate-brown, T., et al. (2010). Opposite effects of delta-nine-tetrahydrocannabinol and cannabidiol on human encephalon function and psychopathology. Neuropsychopharmacology 35, 764–774. doi: x.1038/npp.2009.184
PubMed Abstruse | CrossRef Total Text | Google Scholar
Bisogno, T., Hanus, L., De Petrocellis, Fifty., Tchilibon, S., Ponde, D. East., Brandi, I., et al. (2001). Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br. J. Pharmacol. 134, 845–852. doi: ten.1038/sj.bjp.0704327
PubMed Abstract | CrossRef Total Text | Google Scholar
Bornheim, Fifty. Thousand., and Grillo, Thou. P. (1998). Characterization of cytochrome P450 3A inactivation past cannabidiol-hydrozyquinone as a P450 inactivator. Chem. Res. Toxicol. xi, 1209–1216. doi: 10.1021/tx9800598
PubMed Abstract | CrossRef Full Text
Borrelli, F., Fasolino, I., Romano, B., Capasso, R., Maiello, F., Coppola, D., et al. (2013). Beneficial effect of the not-psychotropic found cannabinoid cannabigerol on experimental inflammatory bowel disease. Biochem. Pharmacol. 85, 1306–1316. doi: 10.1016/j.bcp.2013.01.017
PubMed Abstract | CrossRef Full Text | Google Scholar
Breuer, A., Haj, C. G., Fogaça, M. 5., Gomes, F. V., Silva, N. R., Pedrazzi, J. F., et al. (2016). Fluorinated cannabidiol derivatives: enhancement of activeness in mice models predictive of anxiolytic, antidepressant and antipsychotic effects. PLoS Ane 11:e0158779. doi: 10.1371/periodical.pone.0158779
PubMed Abstract | CrossRef Full Text | Google Scholar
Carlini, E. A., Mechoulam, R., and Lander, N. (1975). Anticonvulsant activity of iv oxygenated cannabidiol derivatives. Res. Commun. Chem. Pathol. Pharmacol. 12, i–15.
PubMed Abstract | Google Scholar
Carreras, J., Kirillova, Yard. S., and Echavarren, A. Thousand. (2016). Synthesis of (-)-cannabimovone and structural reassignment of anhydrocannabimovone through aureate(I)-catalyzed cycloisomerization. Angew. Chemie - Int. Ed. 55, 7121–7125. doi: x.1002/anie.201601834
PubMed Abstruse | CrossRef Full Text | Google Scholar
Cascio, K. G., Gauson, L. A., Stevenson, 50. A., Ross, R. A., and Pertwee, R. Chiliad. (2010). Evidence that the institute cannabinoid cannabigerol is a highly potent α ii-adrenoceptor agonist and moderately potent 5HT 1A receptor antagonist. Br. J. Pharmacol. 159, 129–141. doi: 10.1111/j.1476-5381.2009.00515.x
PubMed Abstract | CrossRef Full Text | Google Scholar
Clayden, J., Moran, W. J., Edwards, P. J., and Laplante, S. R. (2009). The challenge of atropisomerism in drug discovery. Angew. Chemie - Int. Ed. 48, 6398–6401. doi: 10.1002/anie.200901719
PubMed Abstract | CrossRef Total Text | Google Scholar
Console-Bram, L., Brailoiu, E., Brailoiu, G. C., Sharir, H., and Abood, 1000. Eastward. (2014). Activation of GPR18 past cannabinoid compounds: a tale of biased agonism. Br. J. Pharmacol. 171, 3908–3917. doi: ten.1111/bph.12746
PubMed Abstruse | CrossRef Total Text | Google Scholar
De Petrocellis, L., Ligresti, A., Moriello, A. S., Allarà, K., Bisogno, T., Petrosino, S., et al. (2011). Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br. J. Pharmacol. 163, 1479–1494. doi: 10.1111/bph.2011.163.issue-vii
PubMed Abstruse | CrossRef Full Text | Google Scholar
De Petrocellis, Fifty., Orlando, P., Moriello, A. S., Aviello, G., Stott, C., Izzo, A. A., et al. (2012). Cannabinoid actions at TRPV channels: effects on TRPV3 and TRPV4 and their potential relevance to gastrointestinal inflammation. Acta Physiol. 204, 255–266. doi: 10.1111/j.1748-1716.2011.02338.x
PubMed Abstract | CrossRef Full Text | Google Scholar
Devinsky, O., Marsh, E., Friedman, D., Thiele, E., Laux, Fifty., Sullivan, J., et al. (2015). Cannabidiol in patients with handling-resistant epilepsy: an open-characterization interventional trial. Lancet Neurol. 15, 270–278. doi: x.1016/S1474-4422(15)00379-viii
PubMed Abstract | CrossRef Full Text | Google Scholar
El-Alfy, A. T., Ivey, K., Robinson, K., Safwat, A., Radwan, M., Slade, D., et al. (2010). Antidepressant-like issue of Δ9 -tetrahydrocannabinol and other cannabinoids isolated from Cannabis sativa 50. Pharmacol. Biochem. Behav. 95, 434–442. doi: ten.1016/j.pestbp.2011.02.012.Investigations
CrossRef Total Text | Google Scholar
Elmes, M. W., Kaczocha, One thousand., Berger, Westward. T., Leung, Chiliad., Ralph, B. P., Wang, L., et al. (2015). Fatty acid-bounden proteins (FABPs) are intracellular carriers for Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD). J. Biol. Chem. 290, 8711–8721. doi: 10.1074/jbc.M114.618447
PubMed Abstruse | CrossRef Full Text | Google Scholar
ElSohly, One thousand. A., and Gul, W. (2014). "Constituents of cannabis sativa," in Handbook of Cannabis, ed. R. K. Pertwee (Oxford: Oxford University Press), 3–22. doi: x.1093/acprof
CrossRef Full Text | Google Scholar
Esposito, G., Scuderi, C., Valenza, M., Togna, M. I., Latina, V., de Filippis, D., et al. (2011). Cannabidiol reduces Aβ-induced neuroinflammation and promotes hippocampal neurogenesis through PPARγ interest. PLoS ONE 6:e28668. doi: 10.1371/journal.pone.0028668
PubMed Abstruse | CrossRef Full Text | Google Scholar
Fasinu, P. Southward., Phillips, Southward., Elsohly, Chiliad. A., and Walker, L. A. (2016). Current status and prospects for cannabidiol preparations as new therapeutic agents. Pharmacotherapy 36, 781–796. doi: x.1111/j.1875-9114.2016.01780.10
PubMed Abstract | CrossRef Full Text | Google Scholar
Fernandez-Ruiz, J., Sagredo, O., Pazos, Thousand. R., Garcia, C., Pertwee, R., Mechoulam, R., et al. (2013). Cannabidiol for neurodegenerative disorders: important new clinical applications for this phytocannabinoid? Br. J. Clin. Pharmacol. 75, 323–333. doi: 10.1111/j.1365-2125.2012.04341.x
PubMed Abstract | CrossRef Full Text | Google Scholar
Flos, Grand., Lameiras, P., Denhez, C., Mirand, C., and Berber, H. (2016). Atropisomerism virtually Aryl-C(sp3) Bonds: conformational behavior of substituted phenylcyclohexanes in solution. J. Org. Chem. 81, 2372–2382. doi: ten.1021/acs.joc.5b02856
PubMed Abstract | CrossRef Full Text | Google Scholar
Ford, L. A., Roelofs, A. J., Anavi-Goffer, S., Mowat, L., Simpson, D. G., Irving, A. J., et al. (2010). A role for L-blastoff-lysophosphatidylinositol and GPR55 in the modulation of migration, orientation and polarization of human breast cancer cells. Br. J. Pharmacol. 160, 762–771. doi: 10.1111/j.1476-5381.2010.00743.x
PubMed Abstract | CrossRef Total Text | Google Scholar
Fride, E., Feigin, C., Ponde, D. Eastward., Breuer, A., Hanuš, Fifty., Arshavsky, N., et al. (2004). (+)-Cannabidiol analogues which bind cannabinoid receptors merely exert peripheral action just. Eur. J. Pharmacol. 506, 179–188. doi: 10.1016/j.ejphar.2004.10.049
PubMed Abstract | CrossRef Full Text | Google Scholar
Fride, E., Ponde, D., Breuer, A., and Hanuš, L. (2005). Peripheral, just not fundamental effects of cannabidiol derivatives: mediation by CB1 and unidentified receptors. Neuropharmacology 48, 1117–1129. doi: x.1016/j.neuropharm.2005.01.023
PubMed Abstract | CrossRef Full Text | Google Scholar
Gaoni, Y., and Mechoulam, R. (1971). The isolation and structure of delta-1-tetrahydrocannabinol and other neutral cannabinoids from hashish. J. Am. Chem. Soc. 93, 217–224. doi: 10.1021/ja00730a036
PubMed Abstruse | CrossRef Full Text | Google Scholar
Gertsch, J., Pertwee, R. Grand., and Di Marzo, 5. (2010). Phytocannabinoids beyond the cannabis plant - do they be? Br. J. Pharmacol. 160, 523–529. doi: x.1111/j.1476-5381.2010.00745.ten
PubMed Abstruse | CrossRef Full Text | Google Scholar
Gonca, E., and Darıcı, F. (2014). The result of cannabidiol on ischemia/reperfusion-induced ventricular arrhythmias: the role of adenosine A1 receptors. J. Cardiovasc. Pharmacol. Ther. 1:76. doi: 10.1177/1074248414532013
PubMed Abstruse | CrossRef Full Text | Google Scholar
Haj, C. G., Sumariwalla, P. F., Hanuš, L., Kogan, N. M., Yektin, Z., Mechoulam, R., et al. (2015). HU-444, a novel, potent anti-inflammatory, nonpsychotropic cannabinoid. J. Pharmacol. Exp. Ther. 355, 66–75. doi: x.1124/jpet.115.226100
PubMed Abstract | CrossRef Total Text | Google Scholar
Hanus, Fifty., Breuer, A., Tchilibon, S., Shiloah, S., Goldenberg, D., Horowitz, 1000., et al. (1999). HU-308: a specific agonist for CB(ii), a peripheral cannabinoid receptor. Proc. Natl. Acad. Sci. U.S.A. 96, 14228–14233. doi: 10.1073/pnas.96.25.14228
CrossRef Full Text | Google Scholar
Hanuš, 50. O., Meyer, S. Chiliad., Muñoz, East., Taglialatela-Scafati, O., and Appendino, G. (2016). Phytocannabinoids: a unified disquisitional inventory. Nat. Prod. Rep. 33, 1357–1392. doi: ten.1039/c6np00074f
PubMed Abstract | CrossRef Full Text | Google Scholar
Hanus, 50. O., Tchilibon, S., Ponde, D. E., Breuer, A., Fride, E., and Mechoulam, R. (2005). Enantiomeric cannabidiol derivatives: synthesis and binding to cannabinoid receptors. Org. Biomol. Chem. three, 1116–1123. doi: 10.1039/b416943c
PubMed Abstract | CrossRef Full Text | Google Scholar
Hartsel, South. C., Loh, W. H., and Robertson, Fifty. West. (1983). Biotransformation of cannabidiol to cannabielsoin by pause cultures of Cannabis sativa and Saccharum officinarum. Planta Med. 48, 17–nineteen. doi: 10.1055/s-2007-969870
PubMed Abstract | CrossRef Full Text | Google Scholar
Hendricks, H., Malingré, T. M., Batterman, South., and Bos, R. (1978). The essential oil of Cannabis sativa. Pharm. Weekbl. 113, 413–424.
Google Scholar
Colina, A. J., Mercier, M. Due south., Colina, T. D. G., Glyn, South. E., Jones, Due north. A., Yamasaki, Y., et al. (2012). Cannabidivarin is anticonvulsant in mouse and rat. Br. J. Pharmacol. 167, 1629–1642. doi: 10.1111/j.1476-5381.2012.02207.10
PubMed Abstract | CrossRef Total Text | Google Scholar
Colina, T. D. M., Cascio, M. G., Romano, B., Duncan, M., Pertwee, R. G., Williams, C. M., et al. (2013). Cannabidivarin-rich cannabis extracts are anticonvulsant in mouse and rat via a CB1 receptor-independent mechanism. Br. J. Pharmacol. 170, 679–692. doi: 10.1111/bph.12321
PubMed Abstract | CrossRef Total Text | Google Scholar
Huang, Q., Wang, Q., Zheng, J., Zhang, J., Pan, X., and She, X. (2007). A general road to v,6-seco-hexahydrodibenzopyrans and analogues: starting time full synthesis of (+)-Machaeridiol B and (+)-Machaeriol B. Tetrahedron 63, 1014–1021. doi: x.1016/j.tet.2006.10.067
CrossRef Full Text | Google Scholar
Ibeas Bih, C., Chen, T., Nunn, A. V. W., Bazelot, One thousand., Dallas, M., and Whalley, B. J. (2015). Molecular targets of cannabidiol in neurological disorders. Neurotherapeutics 12, 699–730. doi: ten.1007/s13311-015-0377-3
PubMed Abstruse | CrossRef Full Text | Google Scholar
Johns, D. Thou., Behm, D. J., Walker, D. J., Ao, Z., Shapland, East. M., Daniels, D. A., et al. (2007). The novel endocannabinoid receptor GPR55 is activated by atypical cannabinoids but does non mediate their vasodilator furnishings. Br. J. Pharmacol. 152, 825–831. doi: 10.1038/sj.bjp.0707419
PubMed Abstruse | CrossRef Full Text | Google Scholar
Jones, North. A., and Whalley, B. J. (2015). Progress report on new antiepileptic drugs: a summary of the Twelfth Eilat Conference (EILAT XII). Epilepsy Res. 111, 113–114. doi: 10.1016/j.eplepsyres.2015.01.001
PubMed Abstract | CrossRef Full Text
Juknat, A., Kozela, East., Kaushansky, N., Mechoulam, R., and Vogel, Z. (2016). Anti-inflammatory effects of the cannabidiol derivative dimethylheptyl-cannabidiol – studies in BV-2 microglia and encephalitogenic T cells. J. Basic Clin. Physiol. Pharmacol. 27, 289–296. doi: 10.1515/jbcpp-2015-0071
PubMed Abstract | CrossRef Full Text | Google Scholar
Kinney, Westward. A., McDonnell, M. E., Zhong, H. Yard., Liu, C., Yang, Fifty., Ling, W., et al. (2016). Discovery of KLS-13019, a cannabidiol-derived neuroprotective agent, with improved potency, safety, and permeability. ACS Med. Chem. Lett. 7, 424–428. doi: 10.1021/acsmedchemlett.6b00009
PubMed Abstract | CrossRef Full Text | Google Scholar
Kogan, N. G., Rabinowitz, R., Levi, P., Gibson, D., Sandor, P., Schlesinger, G., et al. (2004). Synthesis and antitumor activeness of quinonoid derivatives of cannabinoids. J. Med. Chem. 47, 3800–3806. doi: ten.1021/jm040042o
PubMed Abstract | CrossRef Full Text | Google Scholar
Kogan, North. Grand., Schlesinger, M., Priel, E., Rabinowitz, R., Berenshtein, E., Chevion, K., et al. (2007). HU-331, a novel cannabinoid-based anticancer topoisomerase II inhibitor. Mol. Cancer Ther. half dozen, 173–183. doi: 10.1158/1535-7163.MCT-06-0039
PubMed Abstract | CrossRef Full Text | Google Scholar
Kozela, E., Haj, C., Hanuš, L., Chourasia, M., Shurki, A., Juknat, A., et al. (2015). HU-446 and HU-465, derivatives of the non-psychoactive cannabinoid cannabidiol, decrease the activation of encephalitogenic T cells. Chem. Biol. Drug Des. 87, 143–153. doi: 10.1111/cbdd.12637
PubMed Abstract | CrossRef Full Text | Google Scholar
Krohn, R. Thousand., Parsons, S. A., Fichna, J., Patel, G. D., Yates, R. Thou., Sharkey, Grand. A., et al. (2016). Abnormal cannabidiol attenuates experimental colitis in mice, promotes wound healing and inhibits neutrophil recruitment. J. Inflamm. 13:21. doi: 10.1186/s12950-016-0129-0
PubMed Abstruse | CrossRef Full Text | Google Scholar
Lafuente, H., Alvarez, F. J., Pazos, M. R., Alvarez, A., Rey-Santano, 1000. C., Mielgo, Five., et al. (2011). Cannabidiol reduces encephalon damage and improves functional recovery after acute hypoxia-ischemia in newborn pigs. Pediatr. Res 70, 272–277. doi: 10.1203/PDR.0b013e3182276b11
PubMed Abstract | CrossRef Full Text | Google Scholar
Laprairie, R. B., Bagher, A. M., Kelly, Thousand. Eastward. Thou., and Denovan-Wright, E. M. (2015). Cannabidiol is a negative allosteric modulator of the blazon 1 cannabinoid receptor. Br. J. Pharmacol. 172, 4790–4805. doi: 10.1111/bph.13250
PubMed Abstract | CrossRef Full Text | Google Scholar
Lauckner, J. Eastward., Jensen, J. B., Chen, H.-Y., Lu, H.-C., Hille, B., and Mackie, K. (2008). GPR55 is a cannabinoid receptor that increases intracellular calcium and inhibits Grand current. Proc. Natl. Acad. Sci. U.S.A. 105, 2699–2704. doi: 10.1073/pnas.0711278105
PubMed Abstract | CrossRef Total Text | Google Scholar
Leite, J. R., Carlini, Eastward. A., Lander, Northward., and Mechoulam, R. (1982). Anticonvulsant effects of the (-) and (+)isomers of cannabidiol and their dimethylheptyl homologs. Pharmacology 24, 141–146. doi: 10.1159/000137588
PubMed Abstract | CrossRef Full Text | Google Scholar
Leweke, F. M., Piomelli, D., Pahlisch, F., Muhl, D., Gerth, C. Westward., Hoyer, C., et al. (2012). Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl. Psychiatry 2:e94. doi: 10.1038/tp.2012.15
PubMed Abstract | CrossRef Full Text | Google Scholar
Li, K., Feng, J., Li, Y., Yuece, B., Lin, X., Yu, 50., et al. (2013). Anti-inflammatory office of cannabidiol and O-1602 in cerulein-induced acute pancreatitis in mice. Pancreas 42, 123–129. doi: 10.1097/MPA.0b013e318259f6f0
PubMed Abstract | CrossRef Full Text | Google Scholar
Ligresti, A. (2006). Antitumor activeness of plant cannabinoids with emphasis on the effect of cannabidiol on man breast carcinoma. J. Pharmacol. Exp. Ther. 318, 1375–1387. doi: 10.1124/jpet.106.105247
PubMed Abstruse | CrossRef Total Text | Google Scholar
Maione, S., Piscitelli, F., Gatta, Fifty., Vita, D., De Petrocellis, 50., Palazzo, Due east., et al. (2011). Non-psychoactive cannabinoids modulate the descending pathway of antinociception in anaesthetized rats through several mechanisms of activity. Br. J. Pharmacol. 162, 584–596. doi: ten.1111/j.1476-5381.2010.01063.x
PubMed Abstruse | CrossRef Full Text | Google Scholar
Matsuda, Fifty. A., Lolait, S. J., Brownstein, K. J., Immature, A. C., and Bonner, T. I. (1990). Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346, 561–564. doi: ten.1038/346561a0
PubMed Abstract | CrossRef Total Text | Google Scholar
McAllister, S. D., Murase, R., Christian, R. T., Lau, D., Zielinski, A. J., Allison, J., et al. (2011). Pathways mediating the effects of cannabidiol on the reduction of chest cancer cell proliferation, invasion, and metastasis. Breast Cancer Res. Treat. 129, 37–47. doi: 10.1007/s10549-010-1177-4
PubMed Abstract | CrossRef Full Text | Google Scholar
McHugh, D., Page, J., Dunn, E., and Bradshaw, H. B. (2012). Δ9-Tetrahydrocannabinol and N-arachidonyl glycine are total agonists at GPR18 receptors and induce migration in human endometrial HEC-1B cells. Br. J. Pharmacol. 165, 2414–2424. doi: ten.1111/j.1476-5381.2011.01497.x
PubMed Abstruse | CrossRef Full Text | Google Scholar
McHugh, D., Roskowski, D., Xie, Southward., and Bradshaw, H. B. (2014). Δ9-THC and Due north-arachidonoyl glycine regulate BV-ii microglial morphology and cytokine release plasticity: implications for signaling at GPR18. Front. Pharmacol. iv:162. doi: 10.3389/fphar.2013.00162
CrossRef Full Text | Google Scholar
McKillop, A. M., Moran, B. M., Abdel-Wahab, Y. H. A., Gormley, N. Thou., and Flatt, P. R. (2016). Metabolic furnishings of orally administered minor-molecule agonists of GPR55 and GPR119 in multiple depression-dose streptozotocin-induced diabetic and incretin-receptor-knockout mice. Diabetologia 59, 2674–2685. doi: 10.1007/s00125-016-4108-z
PubMed Abstract | CrossRef Full Text | Google Scholar
McPartland, J. Yard., Drinking glass, M., and Pertwee, R. K. (2007). Meta-analysis of cannabinoid ligand binding affinity and receptor distribution: interspecies differences. Br. J. Pharmacol. 152, 583–593. doi: 10.1038/sj.bjp.0707399
PubMed Abstract | CrossRef Full Text | Google Scholar
Mechoulam, R., Ben-Zvi, Z., and Gaoni, Y. (1968). Hashish-XIII. On the nature of the beam test. Tetrahedron 24, 5615–5624. doi: 10.1016/0040-4020(68)88159-ane
CrossRef Total Text | Google Scholar
Mechoulam, R., Feigenbaum, J. J., Lander, North., Segal, M., Hiltunen, T. U. C., and Consroe, P. (1988). Enantiomeric cannabinoids: stereospecificity of psychotropic activity. Experientia 44, 762–764. doi: 10.1007/BF01959156
CrossRef Full Text | Google Scholar
Mechoulam, R., and Hanuš, Fifty. (2002). Cannabidiol: an overview of some chemic and pharmacological aspects, Part I: Chemic aspects. Chem. Phys. Lipids 121, 35–43. doi: 10.1016/S0009-3084(02)00144-5
PubMed Abstract | CrossRef Full Text | Google Scholar
Mechoulam, R., Hanuš, Fifty. O., Pertwee, R., and Howlett, A. C. (2014). Early phytocannabinoid chemistry to endocannabinoids and across. Nat. Rev. Neurosci. xv, 757–764. doi: ten.1038/nrn3811
PubMed Abstruse | CrossRef Full Text | Google Scholar
Mechoulam, R., Kogan, N. G., Gallily, R., and Breuer, A. (2008). Novel Cannabidiol Derivatives and their apply as anti-inflammatory agents. ternational Patent No WO2008/107879. Washington, DC.
Mechoulam, R., Lander, Northward., Breuer, A., and Zahalka, J. (1990). Synthesis of the individual, pharmacologically singled-out enantiomers of a tetrahydrocannabinol derivative. Tetrahedron Asymmetry 1, 315–318. doi: 10.1016/S0957-4166(00)86322-3
CrossRef Full Text | Google Scholar
Morales, P., Goya, P., Jagerovic, N., and Hernandez-Folgado, L. (2016). Allosteric modulators of the CB1 cannabinoid receptor: a structural update review. Cannabis Cannabinoid Res. one, 22–xxx. doi: 10.1089/tin.2015.0005
CrossRef Total Text | Google Scholar
Morales, P., Hurst, D. P., and Reggio, P. H. (2017). "Molecular targets of the phytocannabinoids: a circuitous picture," in Progress in the Chemical science of Organic Natural Products: Phytocannabinoids, Unravelling the Circuitous Chemistry and Pharmacology of Cannabis sativa, eds A. D. Kinghorn, H. Falk, S. Gibbons, and J. Kobayashi (Berlin: Springer), doi: 10.1007/978-3-319-45541-9_4
CrossRef Full Text | Google Scholar
Mori, M. A., Meyer, Eastward., Soares, 50. Thou., Milani, H., Guimarães, F. S., and de Oliveira, R. M. W. (2017). Cannabidiol reduces neuroinflammation and promotes neuroplasticity and functional recovery later on brain ischemia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 75, 94–105. doi: 10.1016/j.pnpbp.2016.eleven.005
PubMed Abstract | CrossRef Total Text | Google Scholar
Muhammad, I., Li, X.-C., Jacob, M. R., Tekwani, B. L., Dunbar, D. C., and Ferreira, D. (2003). Antimicrobial and antiparasitic (+)- trans -hexahydrodibenzopyrans and analogues from Machaerium multiflorum. J. Nat. Prod. 66, 804–809. doi: ten.1021/np030045o
PubMed Abstract | CrossRef Full Text | Google Scholar
Naftali, T., Mechulam, R., Marii, A., Gabay, 1000., Stein, A., Bronshtain, M., et al. (2017). Low-dose cannabidiol is safe merely non effective in the treatment for Crohn'south disease, a randomized controlled trial. Dig. Dis. Sci. 62, 1–6. doi: x.1007/s10620-017-4540-z
PubMed Abstract | CrossRef Full Text | Google Scholar
Ofek, O., Karsak, M., Leclerc, N., Fogel, M., Frenkel, B., Wright, One thousand., et al. (2006). Peripheral cannabinoid receptor, CB2, regulates bone mass. Proc. Natl. Aacd. Sci. U.s.A. 103, 696–701. doi: 10.1021/jm4005626
PubMed Abstruse | CrossRef Full Text | Google Scholar
Oka, S., Nakajima, Yard., Yamashita, A., Kishimoto, South., and Sugiura, T. (2007). Identification of GPR55 every bit a lysophosphatidylinositol receptor. Biochem. Biophys. Res. Commun. 362, 928–934. doi: 10.1016/j.bbrc.2007.08.078
PubMed Abstract | CrossRef Total Text | Google Scholar
O'Sullivan, S. East., Sun, Y., Bennett, A. J., Randall, M. D., and Kendall, D. A. (2009). Time-dependent vascular actions of cannabidiol in the rat aorta. Eur. J. Pharmacol. 612, 61–68. doi: 10.1016/j.ejphar.2009.03.010
PubMed Abstract | CrossRef Full Text | Google Scholar
Pertwee, R. G., Ross, R. A., Craib, S. J., and Thomas, A. (2002). (-)-Cannabidiol antagonizes cannabinoid receptor agonists and noradrenaline in the mouse vas deferens. Eur. J. Pharmacol. 456, 99–106. doi: 10.1016/S0014-2999(02)02624-9
PubMed Abstruse | CrossRef Total Text | Google Scholar
Pertwee, R. One thousand., Thomas, A., Stevenson, L. A., Maor, Y., and Mechoulam, R. (2005). Testify that (-)-seven-hydroxy-4′-dimethylheptyl-cannabidiol activates a non-CB1, non-CB2, not-TRPV1 target in the mouse vas deferens. Neuropharmacology 48, 1139–1146. doi: 10.1016/j.neuropharm.2005.01.010
PubMed Abstract | CrossRef Full Text | Google Scholar
Peters, Yard., and Kogan, N. G. (2007). HU-331: a cannabinoid quinone, with uncommon cytotoxic properties and low toxicity. Expert Opin. Investig. Drugs 16, 1405–1413. doi: ten.1517/13543784.xvi.9.1405
PubMed Abstract | CrossRef Full Text | Google Scholar
Petronzi, C., Festa, M., Peduto, A., Castellano, M., Marinello, J., Massa, A., et al. (2013). Cyclohexa-2,5-diene-1,4-dione-based antiproliferative agents?: pattern, synthesis, and cytotoxic evaluation. J. Exp. Clin. Cancer Res. 32:24. doi: 10.1186/1756-9966-32-24
PubMed Abstract | CrossRef Full Text | Google Scholar
Pucci, K., Rapino, C., Di Francesco, A., Dainese, E., D'Addario, C., and Maccarrone, M. (2013). Epigenetic control of skin differentiation genes past phytocannabinoids. Br. J. Pharmacol. 170, 581–591. doi: 10.1111/bph.12309
PubMed Abstruse | CrossRef Full Text | Google Scholar
Rajesh, M., Mukhopadhyay, P., Batkai, South., Hasko, G., Liaudet, L., Huffman, J. Westward., et al. (2007a). CB2-receptor stimulation attenuates TNF-alpha-induced homo endothelial cell activation, transendothelial migration of monocytes, and monocyte-endothelial adhesion. Am. J. Physiol. Centre Circ. Physiol. 293, H2210–H2218. doi: ten.1152/ajpheart.00688.2007
PubMed Abstruse | CrossRef Full Text | Google Scholar
Rajesh, 1000., Pan, H., Mukhopadhyay, P., Bátkai, South., Osei-Hyiaman, D., Haskó, G., et al. (2007b). Cannabinoid-2 receptor agonist HU-308 protects against hepatic ischemia/reperfusion injury past attenuating oxidative stress, inflammatory response, and apoptosis. J. Leukoc. Biol. 82, 1382–1389. doi: 10.1189/jlb.0307180
PubMed Abstract | CrossRef Full Text | Google Scholar
Razdan, R. Thou., Dalzell, H. C., and Handrick, G. R. (1974). Hashis. a simple one-step synthesis of (-)-delta1-tetrahydrocannabinol (THC) from p-mentha-2,eight-dien-1-ol and olivetol. J. Am. Chem. Soc. 96, 5860–5865. doi: 10.1021/ja00825a026
PubMed Abstract | CrossRef Full Text | Google Scholar
Reggio, P. H., Bramblett, R. D., Yuknavich, H., Seltzman, H. H., Fleming, D. N., Fernando, Southward. R., et al. (1995). The design, synthesis and testing of desoxy-CBD: further show for a region of steric interference at the cannabinoid receptor. Life Sci. 56, 2025–2032. doi: ten.1016/0024-3205(95)00185-9
PubMed Abstruse | CrossRef Full Text | Google Scholar
Renard, J., Norris, C., Rushlow, Due west., and Laviolette, South. R. (2017). Neuronal and molecular effects of cannabidiol on the mesolimbic dopamine system: implications for novel schizophrenia treatments. Neurosci. Biobehav. Rev. 75, 157–165. doi: 10.1016/j.neubiorev.2017.02.006
PubMed Abstract | CrossRef Total Text | Google Scholar
Ribeiro, A., Almeida, V. I., Costola-de-Souza, C., Ferraz-de-Paula, V., Pinheiro, Yard. L., Vitoretti, 50. B., et al. (2015). Cannabidiol improves lung function and inflammation in mice submitted to LPS-induced acute lung injury. Immunopharmacol. Immunotoxicol. 37, 35–41. doi: 10.3109/08923973.2014.976794
PubMed Abstract | CrossRef Full Text | Google Scholar
Robert, J. J., Ch, L., Ludwig Bercht, C. A., van Ooyen, R., and Spronck, H. J. W. (1977). Cannabinodiol: conclusive identification and synthesis of a new cannabinoid from Cannabis sativa. Phytochemistry 16, 595–597. doi: ten.1016/0031-9422(77)80023-X
CrossRef Full Text | Google Scholar
Stone, Due east. G., Bolognini, D., Limebeer, C. Fifty., Cascio, M. G., Anavi-Goffer, Southward., Fletcher, P. J., et al. (2012). Cannabidiol, a nonpsychotropic component of cannabis, attenuates vomiting and nausea-similar behaviour via indirect agonism of five-HT 1A somatodendritic autoreceptors in the dorsal raphe nucleus. Br. J. Pharmacol. 165, 2620–2634. doi: 10.1111/j.1476-5381.2011.01621.10
PubMed Abstract | CrossRef Total Text | Google Scholar
Romero-Zerbo, Due south. Y., Rafacho, A., Díaz-Arteaga, A., Suárez, J., Quesada, I., Imbernon, 1000., et al. (2011). Role for the putative cannabinoid receptor GPR55 in the islets of Langerhans. J. Endocrinol. 211, 177–185. doi: 10.1530/JOE-11-0166
PubMed Abstract | CrossRef Full Text | Google Scholar
Rosenthaler, Due south., Pöhn, B., Kolmanz, C., Nguyen Huu, C., Krewenka, C., Huber, A., et al. (2014). Differences in receptor binding affinity of several phytocannabinoids do non explain their effects on neural cell cultures. Neurotoxicol. Teratol. 46, 49–56. doi: 10.1016/j.ntt.2014.09.003
PubMed Abstract | CrossRef Full Text | Google Scholar
Ruhaak, Fifty. R., Felth, J., Karlsson, P. C., Rafter, J. J., Verpoorte, R., and Bohlin, Fifty. (2011). Evaluation of the cyclooxygenase inhibiting effects of six major cannabinoids isolated from Cannabis sativa. Biol. Pharm. Bull. 34, 774–778. doi: 10.1248/bpb.34.774
PubMed Abstract | CrossRef Full Text | Google Scholar
Ruiz-Valdepeñas, L., Martínez-Orgado, J. A., Benito, C., Millán, A., Tolón, R. M., and Romero, J. (2011). Cannabidiol reduces lipopolysaccharide-induced vascular changes and inflammation in the mouse brain: an intravital microscopy report. J. Neuroinflammation 8:5. doi: x.1186/1742-2094-8-5
PubMed Abstract | CrossRef Full Text | Google Scholar
Russo, Due east. B., Burnett, A., Hall, B., and Parker, 1000. K. (2005). Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem. Res. 30, 1037–1043. doi: x.1007/s11064-005-6978-i
PubMed Abstract | CrossRef Full Text | Google Scholar
Ryberg, E., Larsson, Northward., Sjögren, Due south., Hjorth, S., Hermansson, N.-O., Leonova, J., et al. (2007). The orphan receptor GPR55 is a novel cannabinoid receptor. Br. J. Pharmacol. 152, 1092–1101. doi: 10.1038/sj.bjp.0707460
PubMed Abstract | CrossRef Full Text | Google Scholar
Schier, A., Ribeiro, North., Coutinho, D., Machado, S., Arias-Carrion, O., Crippa, J., et al. (2014). Antidepressant-similar and anxiolytic-similar effects of cannabidiol: a chemic compound of Cannabis sativa. CNS Neurol. Disord. - Drug Targets xiii, 953–960. doi: ten.2174/1871527313666140612114838
PubMed Abstruse | CrossRef Full Text | Google Scholar
Scuderi, C., Steardo, L., and Esposito, Thousand. (2014). Cannabidiol promotes amyloid forerunner protein ubiquitination and reduction of beta amyloid expression in SHSY5YAPP+ cells through PPARg involvement. Phyther. Res. 28, 1007–1013. doi: 10.1002/ptr.5095
PubMed Abstruse | CrossRef Full Text
Seephonkai, P., Popescu, R., Zehl, G., Krupitza, G., Urban, East., and Kopp, B. (2011). Ferruginenes A-C from rhododendron ferrugineum and their cytotoxic evaluation. J. Nat. Prod. 74, 712–717. doi: x.1021/np100778k
PubMed Abstract | CrossRef Full Text | Google Scholar
Shani, A., and Mechoulam, R. (1974). Cannabielsoic acids: isolation and synthesis by a novel oxidative cyclization. Tetrahedron 30, 2437–2446. doi: 10.1016/S0040-4020(01)97114-5
CrossRef Full Text | Google Scholar
Smoum, R., Baraghithy, S., Chourasia, M., Breuer, A., Mussai, N., Attar-Namdar, Chiliad., et al. (2015). CB2 cannabinoid receptor agonist enantiomers HU-433 and HU-308: an inverse relationship betwixt bounden affinity and biological potency. Proc. Natl. Acad. Sci. The statesA. 112, 8774–8779. doi: 10.1073/pnas.1503395112
PubMed Abstract | CrossRef Total Text | Google Scholar
Stinchcomb, A. L., Golinski, M. J., Hammell, D. C., Howard, J. L., and Banks, Southward. Fifty. (2009). Prodrugs of cannabidiol, compositions comprising prodrugs of cannabidiol, and methods of using the aforementioned. Patent No US2009036523A1. Washington, DC.
Sumariwalla, P. F., Gallily, R., Tchilibon, S., Fride, E., and Mechoulam, R. (2004). A novel synthetic, nonpsychoactive cannabinoid acid ( HU-320 ) with antiinflammatory backdrop in murine collagen-induced arthritis. Arthitis Rheum 50, 985–998. doi: ten.1002/art.20050
PubMed Abstract | CrossRef Total Text | Google Scholar
Taglialatela-Scafati, O., Pagani, A., Scala, F., De Petrocellis, L., Di Marzo, Five., Grassi, G., et al. (2010). Cannabimovone, a cannabinoid with a rearranged terpenoid skeleton from hemp. Eur. J. Org. Chem 2067–2072. doi: 10.1002/ejoc.200901464
CrossRef Full Text | Google Scholar
Takeda, S., Hirayama, A., Urata, Due south., Mano, N., and Aramaki, H. (2011). Cannabidiol-2',6'-dimethyl ether as an constructive protector of 15- lipoxygenase-mediated low-density lipoprotein oxidation in vitro. Biol. Pharm. Bull. 34, 1252–1256. doi: 10.1016/j.drudis.2011.09.009
PubMed Abstruse | CrossRef Total Text | Google Scholar
Takeda, Due south., Hirota, R., Teradaira, S., and Takeda-imoto, M. (2015). Cannabidiol-2',six'-dimethyl ether stimulates torso weight gain in apolipoprotein Eastward-deficient BALB/c, KOR/Stm. J. Toxicol. Sci. 40, 739–743. doi: 10.2131/jts.40.739
PubMed Abstract | CrossRef Full Text | Google Scholar
Takeda, S., Misawa, Thousand., Yamamoto, I., and Watanabe, K. (2008). Cannabidiolic acid as a selective cyclooxygenase-2 inhibitory component in cannabis. Drug Metab. Dispos. 36, 1917–1921. doi: 10.1124/dmd.108.020909
PubMed Abstract | CrossRef Total Text | Google Scholar
Takeda, Due south., Okajima, South., Miyoshi, H., Yoshida, M., Okamoto, Y., Okada, T., et al. (2012). Cannabidiolic acid, a major cannabinoid in cobweb-type cannabis, is an inhibitor of MDA-MB-231 chest cancer cell migration. Toxicol. Lett. 214, 314–319. doi: 10.1016/j.toxlet.2012.08.029
PubMed Abstract | CrossRef Full Text | Google Scholar
Takeda, S., Usami, Due north., Yamamoto, I., and Watanabe, Thousand. (2009). Cannabidiol-2′,six′-dimethyl ether, a cannabidiol derivative, is a highly stiff and selective 15-lipoxygenase inhibitor. Drug Metab. Dispos. 37, 1733–1737. doi: 10.1124/dmd.109.026930
PubMed Abstruse | CrossRef Total Text | Google Scholar
Tanaka, H., Ichino, K., and Ito, K. (1984). A novel dihydrochalcone, linderatin from Lidera Umbellata Var, Lancea. Chem. Pharm. Bull. (Tokyo) 32, 3747–3750. doi: x.1248/cpb.32.3747
CrossRef Full Text | Google Scholar
Tchilibon, Southward., and Mechoulam, R. (2000). Synthesis of a primary metabolite of cannabidiol. Org. Lett. ii, 3301–3303. doi: x.1021/ol006369a
CrossRef Full Text | Google Scholar
Thomas, A., Baillie, Thou. 50., Phillips, A. Grand., Razdan, R. K., Ross, R. A., and Pertwee, R. G. (2007). Cannabidiol displays unexpectedly high authorization as an antagonist of CB1 and CB2 receptor agonists in vitro. Br. J. Pharmacol. 150, 613–623. doi: 10.1038/sj.bjp.0707133
PubMed Abstract | CrossRef Full Text | Google Scholar
Ujváry, I., and Hanuš, L. (2016). Homo metabolites of cannabidiol: a review on their formation, biological activity, and relevance in therapy. Cannabis Cannabinoid Res. 1, ninety–101. doi: x.1089/can.2015.0012
CrossRef Full Text | Google Scholar
Usami, North., Okuda, T., Yoshida, H., Kimura, T., Watanabe, K., Yoshimura, H., et al. (1999). Synthesis and pharmacological evaluation in mice of halogenated cannabidiol derivatives. Chem. Pharm. Balderdash. 47, 1641–1645. doi: 10.1248/cpb.47.1641
PubMed Abstruse | CrossRef Full Text | Google Scholar
Vuolo, F., Petronilho, F., Sonai, B., Ritter, C., Hallak, J. Eastward. C., Zuardi, A. W., et al. (2015). Evaluation of serum cytokines levels and the office of cannabidiol treatment in animal model of asthma. Mediators Inflamm. 2015:538670. doi: 10.1155/2015/538670
PubMed Abstract | CrossRef Full Text | Google Scholar
Whyte, L. Southward., Ryberg, E., Sims, N. A., Ridge, S. A., Mackie, Thou., Greasley, P. J., et al. (2009). The putative cannabinoid receptor GPR55 affects osteoclast part in vitro and bone mass in vivo. Proc. Natl. Acad. Sci. U.Southward.A. 106, 16511–16516. doi: x.1073/pnas.0902743106
PubMed Abstract | CrossRef Full Text | Google Scholar
Wright, South., Sommerville, Chiliad., Jones, N. A., and Whalley, B. J. (2015). Progress report on new antiepileptic drugs : a summary of the twelfth eilat conference (EILAT XII). Epilepsy Res. 111, 111–113. doi: 10.1016/j.eplepsyres.2015.01.001
PubMed Abstract | CrossRef Full Text
Xiong, West., Cui, T., Cheng, K., Yang, F., Chen, S. R., Willenbring, D., et al. (2012). Cannabinoids suppress inflammatory and neuropathic pain by targeting alpha3 glycine receptors. J. Exp. Med. 209, 1121–1134. doi: 10.1084/jem.20120242
PubMed Abstract | CrossRef Total Text | Google Scholar
Yamamoto, I., Gohda, H., Narimatsu, Due south., Watanabe, K., and Yoshimura, H. (1991). Cannabielsoin equally a new metabolite of cannabidiol in mammals. Pharmacol. Biochem. Behav. 40, 541–546. doi: 10.1016/0091-3057(91)90360-Due east
CrossRef Full Text | Google Scholar
Yamaori, S., Okushima, Y., Masuda, Grand., Kushihara, Grand., Katsu, T., Narimatsu, S., et al. (2013). Structural requirements for potent directly inhibition of human cytochrome P450 1A1 by cannabidiol: part of pentylresorcinol moiety. Biol. Pharm. Bull. 36, 1197–1203. doi: 10.1248/bpb.b13-00183
PubMed Abstract | CrossRef Full Text | Google Scholar
Yang, K.-H., Galadari, Due south., Isaev, D., Petroianu, G., Shippenberg, T. S., and Oz, Yard. (2010). The nonpsychoactive cannabinoid cannabidiol inhibits 5-hydroxytryptamine3A receptor-mediated currents in Xenopus laevis oocytes. J. Pharmacol. Exp. Ther. 333, 547–554. doi: 10.1124/jpet.109.162594
PubMed Abstract | CrossRef Total Text | Google Scholar
Yin, H., Chu, A., Li, W., Wang, B., Shelton, F., Otero, F., et al. (2009). Lipid G protein-coupled receptor ligand identification using beta-arrestin Path Hunter assay. J. Biol. Chem. 284, 12328–12338. doi: 10.1074/jbc.M806516200
PubMed Abstract | CrossRef Full Text | Google Scholar
DOWNLOAD HERE
Posted by: darnellthound.blogspot.com
Post a Comment